pDOS
- How we create molecular diversity
- In the search for new therapeutic agents for currently incurable diseases, attention has turned to traditionally “undruggable” targets, and collections of drug-like small molecules with high diversity and quality have become a prerequisite for new breakthroughs.
To generate such collections, the key challenge has become how to design and synthesize drug like small-molecule libraries with improved biological relevancy as well as maximum molecular diversity.
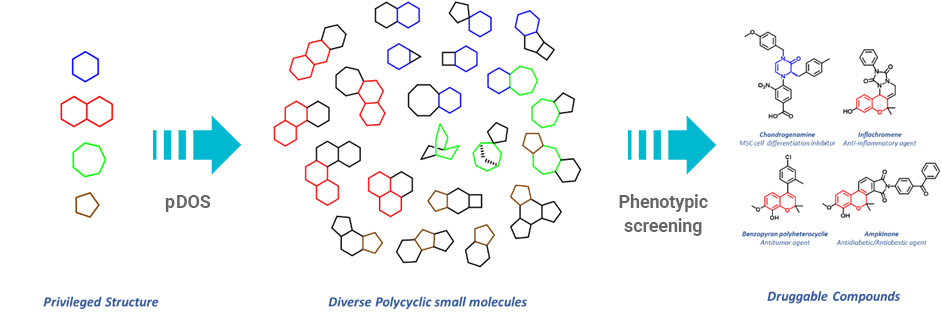
To address these unmet needs for maximizing molecular diversity with high relevance in the biological space, we initiated a ‘‘privileged-substructure-based diversity-oriented synthesis (pDOS) strategy’’ and attempted to emphasize the importance of skeletal diversity through the creative reconstruction of privileged substructures embedded in polyheterocyclic molecular frameworks.
pDOS can generate collections of novel drug-like small molecules with high molecular diversity and biological relevancy that are highly useful for the discovery of novel therapeutic agents.
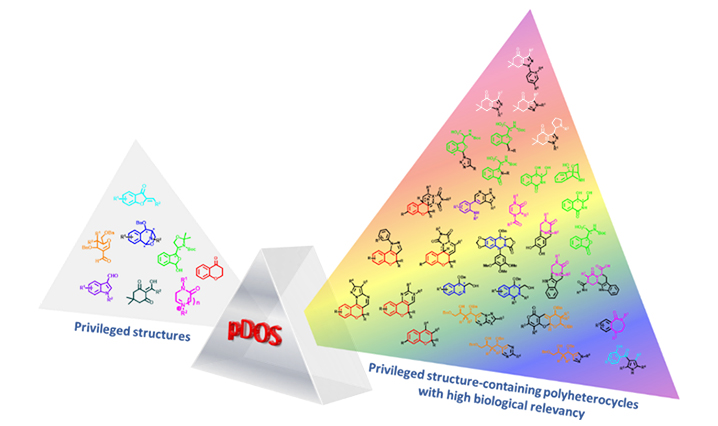
Privileged structures can serve as chemical “navigators” for the efficient construction of biologically relevant small-molecule libraries with diverse structural features. Therefore, the pDOS can be considered as a suitable design strategy for the systematic enhancement of molecular diversity and thus for the discovery of novel chemical entities in chemical biology and drug discovery aimed at modulating diverse biological pathways including traditionally “undruggable” targets.
Publications
-
1.
Nature-inspired remodeling of (aza)indoles to meta-aminoaryl nicotinates for late-stage conjugation of vitamin B3 to (hetero)arylamines. Varun, B.V.†; Vaithegi, K.†; Yi, S.; Park, S.B.* Nature Communications, 2020 11, 6308
-
2.
A divergent synthetic pathway for pyrimidine-embedded medium-sized azacycles through an N-quaternizing strategy. Choi, Y.†; Kim, H.†; Park, S.B.* Chem. Sci., 2019, 10, 569–575
-
3.
Diversity-Oriented Synthetic Strategy for Developing Chemical Modulator of Protein–Protein Interaction. Kim, J.; Koo, J.; Jung, J.; Cho, W.; Lee, W. S.; Kim, C.; Park, W.; Park, S.B.* Nature Communications, 2016 7, 13196.
-
4.
Privileged Structures: Efficient Chemical “Navigators” toward Unexplored Biologically Relevant Chemical Spaces. Kim, J.; Kim, H.; Park, S.B.* J. Am. Chem. Soc. 2014, 136(42), 14629–14638.
-
5.
A Design Strategy for Drug-like Polyheterocycles with Privileged Substructures for Discovery of Specific Small-molecule Modulators. Oh, S.; Park, S.B.* Chem. Commun. 2011 47(48), 12754–12761.
